Why does Carbon form 4 covalent bonds instead of 2?
Most people are not in the slightest bit interested in the answer to this question. For the 1% who are though, this vexing little question is at the heart of organic chemistry - that bitch of subject that seems to drive people alternately mad/ecstatic. I always knew C formed 4 bonds, and got through uni organic chem and some other stuff without really knowing/remembering why. So....years later, I decided to sort myself out, with a particular interest in why the 4 single bonds carbon makes are done with 'sp3' orbitals and why a carbon double bond uses 'sp2' etc. The numbers 3 and 2 didn't seem to have anything to do with anything - but they do, as you can see below.
Here's the answer to why carbon forms 4 covalent bonds instead of 2:
The trivial answer is that a carbon atom that forms 4 covalent bonds is in a lower energy state that one that forms 2. But what happens when this occurs? Carbon's valence shell is configured 2s2 2p2 (remember the p shell can contain up to 6 electrons, in a 3-D 2px, 2py, 2pz shape). Two half-filled p orbitals should mean stable molecules like CH2 - the 2 electrons from the H filling the 2px and 2py subshells. But....these don't normally exist ...instead carbon likes to form 4 covalent bonds with other atoms. Why 4 and not 2? Because the 2s2 2p2 shell hybridizes. Why does it hybridise? Because it results in a lower energy state. After hybridization there are NO LONGER any s or p shells, and we're left with 4 hybrid orbitals that are 'a little bit s and a little bit p'. This is really important to understand. They've gone forever! Why are the 4 hybrid orbitals called 'sp3'? Because the new hybrid effectively replaces 1 s and 3 p orbitals, to give 4 single bonding opportunities.
2s2 2p2 becomes : sp3 sp3 sp3 sp3 (the hybrid).
What's an sp3 shell look like? sp3 shells look nothing like s or p shells...more like a short stocky baseball bat (whereas an s orbital is a sphere, and a p orbital is like 3 double baseball bats without the handles!). They're called sp3 because the s and the 3 p shells combine to make 4 sp3 orbitals. Because there are 4 of them, and they like to be as far away from each other as possible, C forms (single bonding) tetrahedral shapes ie. 4 triangle surface planes.
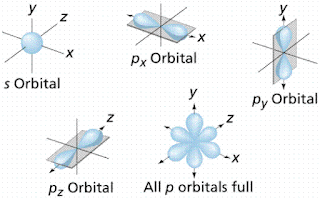
Here's a picture of the unhybridized orbitals (s and p):
And here's a picture of the orbitals hybridizing:
I admit that wasn't a great job explaining one of organic chemistry's most fundamental concepts, but hopefully it helped. The most important thing I realised was that sp3 orbitals are not s and not p. They are completely different, and the old s and p orbitals have gone.
H2C=CH2
1 x s and 2 p orbitals create 3 x sp2 orbitals. The remaining p orbital on the 2 C atoms forms a pi bond. In conjunction with a sigma bond, the pi+sigma form the double bond between the carbons. The hybrids are called sp2 because they're made up of 1 x s orbital, and 2 of the p orbitals (as opposed to 3 of the p orbitals in sp3 hybridization).
A triple bond's no different: 1 x s and 1 x p form an sp orbital. 2 p's then form 2 pi bonds (used for the triple bond in conjunction with the remaining sigma bond). Between the carbon atoms, you've got one sigma bond (from the sp's touching), and 2 pi bonds to make 3! (the sp hybrid has nothing to do with the normal p pi bonding). Easy eh?
Ethyne (acetylene):
Hopefully this explanation also clears up that confusing issue of naming the hybrid orbitals. sp3 for 4 bonds, sp2 for double, and sp for triple never made any sense to me, until I realised they're just the formula that represents how many of the old s and p bonds combined. And a quick note on terminology: the orbitals are named 's', 'p' and 'sp3' etc. but the bonds are called 'sigma' or 'pi', depending on their source.
* A more advanced description is available at: UC Davis - Hybrid Orbitals And a good explanation is also available at: Organic Chemistry (Carey). The Carey book is an excellent resource for organic chemistry.
Here's the answer to why carbon forms 4 covalent bonds instead of 2:
The trivial answer is that a carbon atom that forms 4 covalent bonds is in a lower energy state that one that forms 2. But what happens when this occurs? Carbon's valence shell is configured 2s2 2p2 (remember the p shell can contain up to 6 electrons, in a 3-D 2px, 2py, 2pz shape). Two half-filled p orbitals should mean stable molecules like CH2 - the 2 electrons from the H filling the 2px and 2py subshells. But....these don't normally exist ...instead carbon likes to form 4 covalent bonds with other atoms. Why 4 and not 2? Because the 2s2 2p2 shell hybridizes. Why does it hybridise? Because it results in a lower energy state. After hybridization there are NO LONGER any s or p shells, and we're left with 4 hybrid orbitals that are 'a little bit s and a little bit p'. This is really important to understand. They've gone forever! Why are the 4 hybrid orbitals called 'sp3'? Because the new hybrid effectively replaces 1 s and 3 p orbitals, to give 4 single bonding opportunities.
2s2 2p2 becomes : sp3 sp3 sp3 sp3 (the hybrid).
What's an sp3 shell look like? sp3 shells look nothing like s or p shells...more like a short stocky baseball bat (whereas an s orbital is a sphere, and a p orbital is like 3 double baseball bats without the handles!). They're called sp3 because the s and the 3 p shells combine to make 4 sp3 orbitals. Because there are 4 of them, and they like to be as far away from each other as possible, C forms (single bonding) tetrahedral shapes ie. 4 triangle surface planes.
Here's a picture of the unhybridized orbitals (s and p):
And here's a picture of the orbitals hybridizing:
I admit that wasn't a great job explaining one of organic chemistry's most fundamental concepts, but hopefully it helped. The most important thing I realised was that sp3 orbitals are not s and not p. They are completely different, and the old s and p orbitals have gone.
Carbon and double bonds
How does a double bond work? Think of a carbon double bond in a molecule like ethylene as a carbon with 3 bonds. The hybridization logic is the same as for sp3, but this time we end up with 2 single bonds, and 1 double bond. Using ethylene as an example:H2C=CH2
1 x s and 2 p orbitals create 3 x sp2 orbitals. The remaining p orbital on the 2 C atoms forms a pi bond. In conjunction with a sigma bond, the pi+sigma form the double bond between the carbons. The hybrids are called sp2 because they're made up of 1 x s orbital, and 2 of the p orbitals (as opposed to 3 of the p orbitals in sp3 hybridization).
A triple bond's no different: 1 x s and 1 x p form an sp orbital. 2 p's then form 2 pi bonds (used for the triple bond in conjunction with the remaining sigma bond). Between the carbon atoms, you've got one sigma bond (from the sp's touching), and 2 pi bonds to make 3! (the sp hybrid has nothing to do with the normal p pi bonding). Easy eh?
Ethyne (acetylene):
Hopefully this explanation also clears up that confusing issue of naming the hybrid orbitals. sp3 for 4 bonds, sp2 for double, and sp for triple never made any sense to me, until I realised they're just the formula that represents how many of the old s and p bonds combined. And a quick note on terminology: the orbitals are named 's', 'p' and 'sp3' etc. but the bonds are called 'sigma' or 'pi', depending on their source.
* A more advanced description is available at: UC Davis - Hybrid Orbitals And a good explanation is also available at: Organic Chemistry (Carey). The Carey book is an excellent resource for organic chemistry.




Thank you so much, I am in that 1%, your explanation is crystal clear, is helping me a lot.
ReplyDeleteDelighted to help! I was in the 1% too, or rather the probably-large group of people who really needed this explained to me clearly. I needed to know this as part of a pre-med exam (MCAT), fortunately it got asked on the exam and I vaguely knew what I was talking about. Not that it's relevant when it comes to patients though!
DeleteJust spent some time deleting all the spam rubbish "I loved your post, it was well written" .... then "now let me tell you about my treatment for constipation/crackz for software/on-line gambling" and all the other spam scams designed to rip-off dumb people. This is an article about carbon bonds, not a reference page for get-rich-quick impoverished 3rd-world spammers. So I'll delete them as quickly as I can get to them.
ReplyDeleteGood content. You write beautiful things.
ReplyDeletesportsbet
vbet
vbet
mrbahis
hacklink
hacklink
sportsbet
mrbahis
korsan taksi
Good article text write content successfull... thanks.
ReplyDeletetipobet
slot siteleri
poker siteleri
betmatik
kralbet
kibris bahis siteleri
bonus veren siteler
betpark
başakşehir
ReplyDeletebayrampaşa
beşiktaş
beykoz
beylikdüzü
75K0
mecidiyeköy
ReplyDeletesakarya
istanbul
kayseri
ordu
OFF
adapazarı
ReplyDeleteadıyaman
afyon
alsancak
antakya
OVHRBK
This comment has been removed by the author.
ReplyDeleteŞaşırmadığım bir kalite kendi bedeninizi kesinlikle almalısınız
ReplyDeleteCool and that i have a tremendous present: Where To Remodel House renovate my house
ReplyDeleteشركة تنظيف بالقطيف HLBe0mHQge
ReplyDeleteشركة تسليك مجاري بالقصيم MhcQPRIsR3
ReplyDelete